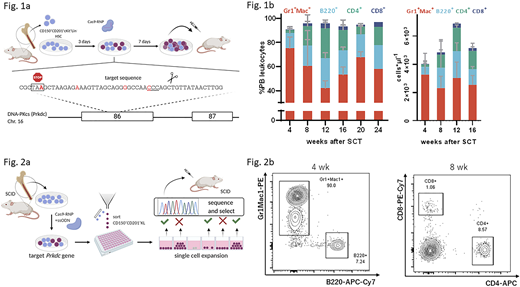
Background
The application of gene editing in hematopoietic stem cells (HSCs) holds great promise for the treatment of genetic blood disorders such as severe combined immunodeficiency (SCID). However, one critical bottleneck is that edited HSCs cannot easily be expanded ex vivo without concomitant loss of self-renewal. This limitation excludes the possibility of growing functional HSCs from single cells, which would enable the selection of desired clones based on sequence verification of relevant on- and off-target modifications. We recently reported on a defined, serum-free, polymer-based culture protocol that selectively facilitates in vitro proliferation of murine HSCs [1]. In the current study, we aimed to expand functional, CRISPR/Cas9-edited HSCs from bulk cell populations as well as from cloned, single HSCs to generate grafts capable of hematopoietic reconstitution. We show that HSCs from the murine PrkdcSCID model, which harbors a point mutation in the Prkdc gene leading to B and T cell deficiency, can be edited and expanded to correct the immunodeficient phenotype after transplantation. Furthermore, we demonstrate that single, gene-edited HSCs can be cloned and expanded using our system to generate functional HSCs for SCT.
Methods
CD150+CD201+c-Kit+Lin- (CD150+CD201+KL) HSCs from C.B17/Icr-PrkdcSCID (SCID) mice were isolated and cultured in polymer-based medium supplemented with recombinant cytokines. Gene editing was performed with Cas9 protein and appropriate gRNAs delivered as ribonucleoprotein complexes (RNPs) via electroporation. HDR donors were supplied as single-strand oligonucleotides (ssODNs). Stem cell transplantations (SCTs) were carried out after lethal irradiation with 2.5 Gy.
Results
To demonstrate that edited HSCs can be expanded as a bulk population and efficiently engraft to correct a disease phenotype, SCID mouse-derived donor HSCs were subjected to Cas9-mediated gene editing at the Prkdc locus (Fig. 1a). Total cells and primitive CD201+CD150+cKit+Lin-(KL) cells expanded 70- and 10-fold, respectively, over seven days after which bulk populations were transplanted into SCID mice. Inference of CRISPR edits (ICE) analysis performed at the time of SCT indicated an HDR frequency of 29%±10%. The emergence of B and T cells in peripheral blood samples was observed from four weeks after transplantation (B220+: 21±7%, CD4+: 27±4%, CD8+: 4±1%; Fig 1b). We also confirmed the presence of B and T cells in the spleen and thymus of transplanted mice. Immunization experiments showed immunoglobulin titer levels equal to healthy control mice after challenge with a T-dependent antigen. We conclude that expansion and autologous SCT of edited HSCs restores a functional B and T cell compartment in SCID mice. We next inquired whether our system could be used to expand single, edited HSC clones. To this end, we sorted single, edited CD150+CD201+KL clones by flow cytometry and expanded them for two weeks. Genomic DNA was sampled from growing colonies and editing outcomes at the Prkdc locus were individually assessed to screen for corrected HSCs (Fig. 2a). After transplantation of the selected clones, B and T cells could be detected starting from 4 weeks and 8 weeks, respectively, in peripheral blood (Fig. 2b), suggesting that functional HSCs could be expanded from edited clones. Interestingly, we found that engraftment was associated with high expression of EPCR in the CD150+KL populations of single cell-derived HSC colonies.
Conclusion
We have shown that functional, Cas9-edited SCID and wildtype HSCs can be expanded in our defined culture system. Corrected SCID HSCs contributed to hematopoietic reconstitution of B and T lineages conferring restored immunity in vivo. Furthermore, we were able to generate transplantable HSCs from single edited clones. This approach has important applications in HSC gene editing and has potential to overcome marker-based selection strategies since individual clones can be interrogated and selected for targeted gene editing events prior to transplantation. Our expansion system will serve as a tool to further the development of targeted gene therapeutic strategies.
[1] Wilkinson et al., Nature 571, 117-121 (2019)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.